DESCARGAR
VERSIÓN EXTENSA
DESCARGAR
ANEXOS
DESCARGAR
VERSIÓN CORTA
DESCARGAR RECOMENDACIONES Y FLUJOGRAMAS
vacio
vacio
Ámbito
- Esta guía debe ser usada en todos los establecimientos del seguro social del Perú (EsSalud), según lo correspondiente a su nivel de atención.
Población y alcance
- Población:Adultos con fibrilación auricular atendidos en EsSalud
- Usuarios: La guía está dirigida al personal médico y no médico, que participa en la atención multidisciplinaria del paciente con fibrilación auricular. Las recomendaciones serán aplicadas por médicos generales, internistas, emergenciólogos, cardiólogos, médicos de familia, cirujanos cardiovasculares, intensivistas, médicos residentes de las diversas especialidades, médicos gestores y enfermeros. Asimismo, podrá ser utilizada como referencia por estudiantes de profesiones relacionadas al ámbito de la salud, personal técnico y pacientes.
Autores
Grupo elaborador
Expertos clínicos:
- Richard Soto Becerra.
Instituto Nacional Cardiovascular INCOR, EsSalud* - Carolina Luisa Guevara Caicedo.
Instituto Nacional Cardiovascular INCOR, EsSalud* - Mario Paul Cabrera Saldaña.
Instituto Nacional Cardiovascular INCOR, EsSalud* - Pío Daniel Zelaya Castro.
Instituto Nacional Cardiovascular INCOR, EsSalud* - Gladys Martha Espinoza Rivas.
Instituto Nacional Cardiovascular INCOR, EsSalud - Ricardo José Zegarra Carhuaz.
Instituto Nacional Cardiovascular INCOR, EsSalud
* Expertos clínicos que participaron en el proceso de actualización de la guía de práctica clínica
Metodólogos:
- José Manuel Montes Alvis.
IETSI, EsSalud* - Percy Fernando Nateros Baldeón.
IETSI, EsSalud* - Liz Pamela Mendoza Aucaruri.
IETSI, EsSalud* - Jessica Hanae Zafra Tanaka.
IETSI, EsSalud - Sergio André Goicochea Lugo.
IETSI, EsSalud - Kevin Arturo Pacheco Barrios.
IETSI, EsSalud - Alvaro Renzo Taype Rondán.
IETSI, EsSalud - Adrián Vladimir Hernández Diaz.
Consultor - José Alejandro Piscoya Rivera.
Consultor
* Metodólogos que participaron en el proceso de actualización de la guía de práctica clínica.
Gestores:
- Stefany Salvador Salvador.
IETSI, EsSalud* - Vladimir Ernesto Santos Sánchez.
IETSI, EsSalud* - Raúl Alonso Timaná Ruiz.
IETSI, EsSalud
* Gestores que participaron en el proceso de actualización de la guía de práctica clínica.
Metodología
Resumen de la metodología:
- Conformación del GEG: La Dirección de Guías de Práctica Clínica, Farmacovigilancia y Tecnovigilancia, del Instituto de Evaluación de Tecnologías en Salud e Investigación (IETSI) del Seguro Social del Perú (EsSalud), conformó un grupo elaborador de la guía (GEG), que incluyó médicos especialistas y metodólogos.
- Planteamiento de preguntas clínicas: En concordancia con los objetivos y alcances de esta GPC, se formularon las preguntas clínicas.
- Búsqueda de la evidencia para cada pregunta: Para cada pregunta clínica, se realizaron búsquedas de revisiones sistemáticas (publicadas como artículos científicos o guías de práctica clínica). De no encontrar revisiones de calidad, se buscaron estudios primarios, cuyo riesgo de sesgo fue evaluado usando herramientas estandarizadas.
- Evaluación de la certeza de la evidencia: Para graduar la certeza de la evidencia, se siguió la metodología Grading of Recommendations Assessment, Development, and Evaluation (GRADE), y se usaron tablas de Summary of Findings (SoF).
- Formulación de las recomendaciones: El GEG revisó la evidencia recolectada para cada una de las preguntas clínicas en reuniones periódicas, en las que formuló las recomendaciones usando la metodología GRADE, otorgándole una fuerza a cada una. Para ello, se tuvo en consideración los beneficios y daños de las opciones, valores y preferencias de los pacientes, aceptabilidad, factibilidad, equidad y uso de recursos. Estos criterios fueron presentados y discutidos, tomando una decisión por consenso o mayoría simple. Asimismo, el GEG emitió puntos de buenas prácticas clínicas (BPC) sin una evaluación formal de la evidencia, y mayormente en base a su experiencia clínica.
- Revisión externa: La presente GPC fue revisada en reuniones con profesionales representantes de otras instituciones, tomadores de decisiones, y expertos externos.
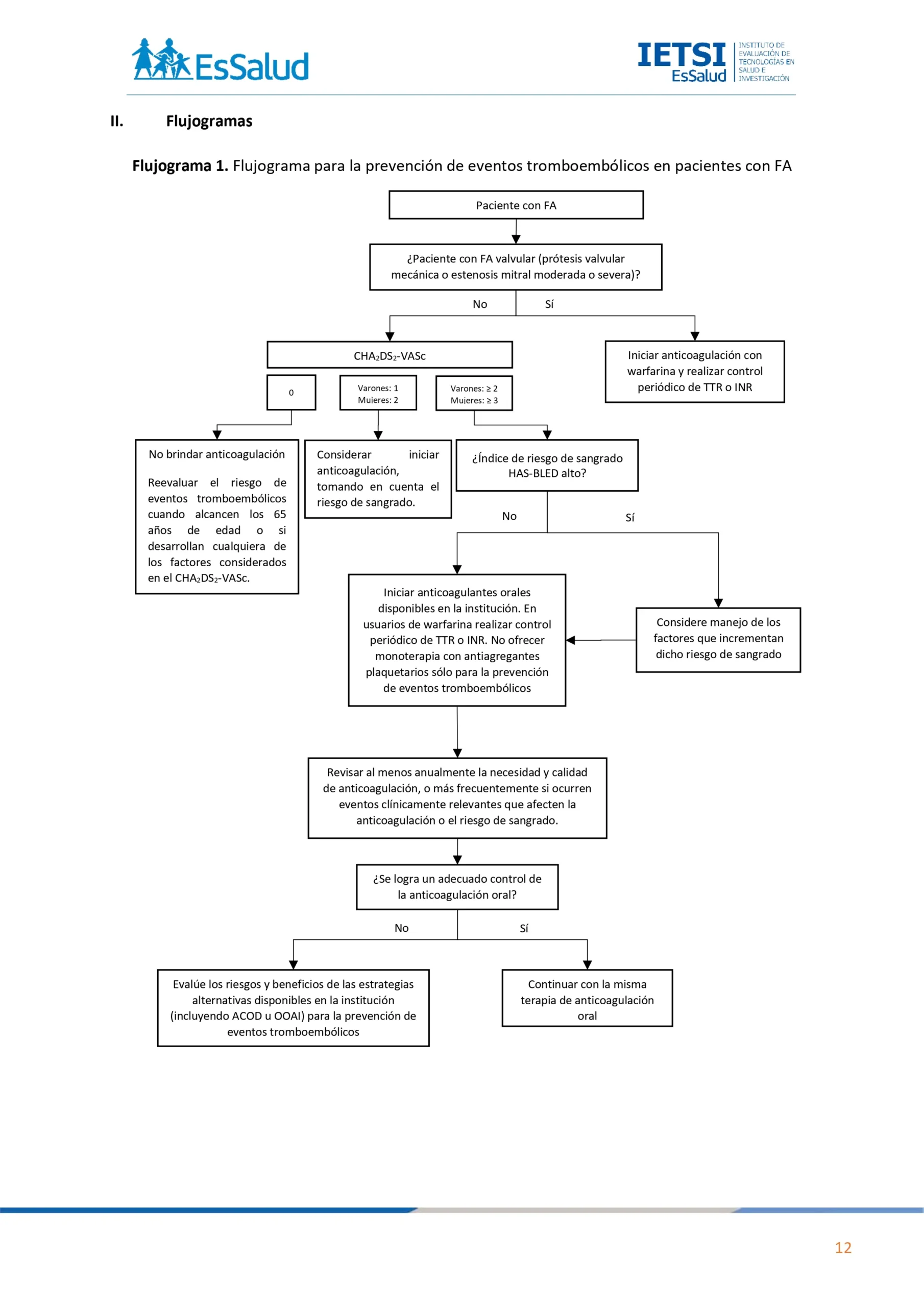
Flujogramas que resumen el contenido de la GPC

vacio
vacio
1. ATRIA vs CHA2DS2-VASc
Descargar PDF con el desarrollo de la pregunta.
Recomendación 1:
En pacientes con FA no valvular, recomendamos usar el índice de riesgo CHA2DS2-VASc para evaluar el riesgo de eventos tromboembólicos.
(Recomendación fuerte a favor, Certeza de la evidencia: Muy baja)
BPC1:
Utilizar el índice de riesgo CHA2DS2-VASc en pacientes con FA no valvular, sin importar la presencia de síntomas y el tipo
(paroxística, persistente o permanente).
2. Terapia antitrombótica
Descargar PDF con el desarrollo de la pregunta.
BPC 1:
En pacientes con FA no valvular y CHA2DS2-VASc de 0 para varones y 1 para mujeres, no ofrecer terapia para prevención de eventos tromboembólicos.
BPC 2:
En varones con FA no valvular y CHA2DS2-VASc de 1, y mujeres con CHA2DS2-VASc de 2, considerar iniciar anticoagulación, luego de una valoración individualizada de los beneficios y daños.
BPC 3:
En varones con FA no valvular y CHA2DS2-VASc de 2 o más, y mujeres con CHA2DS2-VASc de 3 o más, iniciar anticoagulación.
BPC 4:
En pacientes con FA valvular (portadores de prótesis valvular mecánica o padecen de estenosis mitral moderada o severa), iniciar anticoagulación con warfarina sin considerar el puntaje del índice de riesgo CHA2DS2-VASc.
Recomendación 1:
En pacientes con FA no valvular en quienes se decida iniciar anticoagulación, sugerimos emplear el anticoagulante oral disponible en la institución que mejor se adapte a las características clínicas del paciente.
(Recomendación condicional a favor, Certeza de la evidencia: Muy baja)
Recomendación 2:
En pacientes con FA no valvular, recomendamos no ofrecer monoterapia con antiagregantes plaquetarios sólo como medida de prevención de eventos tromboembólicos.
(Recomendación fuerte en contra, Certeza de la evidencia: Muy baja)
BPC 1:
En pacientes usuarios de warfarina, calcule el tiempo en rango terapéutico (TTR) en cada visita. Cuando calcule el TTR:
a. Use un método validado de medida como el método Rosendaal para la dosificación ayudada por computadora o las proporciones de pruebas en rango para las dosis manuales (INR).
b. Excluya las medidas tomadas durante las 6 primeras semanas de tratamiento.
c. Calcule el TTR en un periodo de mantenimiento de al menos 6 meses.
d. Debe realizarse el control del INR en períodos cercanos hasta alcanzar un nivel óptimo y estable, y luego debe ser mensualmente.
e. Cuando se inicia la anticoagulación debe crearse una tarjeta de control de INR única para el seguimiento.
BPC 2:
Reevalúe la anticoagulación con warfarina de un paciente con pobre control de anticoagulación representado por cualquiera de los siguientes:
a. 2 valores de INR mayores de 5, o 1 INR mayor de 8 en los últimos 6 meses.
b. 2 valores de INR menores a 1.5 dentro de los últimos 6 meses.
c. TTR menor a 65%.
BPC 3:
Si el pobre control de anticoagulación con warfarina no se puede mejorar, evalúe los riesgos y beneficios de las estrategias alternativas disponibles en la institución (incluyendo los anticoagulantes orales directos [ACOD] o la oclusión de la orejuela de la aurícula izquierda [OOAI]) para la prevención de eventos tromboembólicos y discútalas con el paciente.
BPC 4:
Para los pacientes que no están tomando anticoagulación, reevalúe el riesgo de eventos tromboembólicos cuando alcancen los 65 años de edad o si desarrollan cualquiera de los siguientes eventos a cualquier edad:
a. Diabetes mellitus
b. Hipertensión arterial
c. Insuficiencia cardiaca
d. Enfermedad arterial periférica
e. Enfermedad coronaria
f. DCV, TIA o tromboembolismo sistémico
BPC 5:
Para los pacientes que no toman anticoagulación debido al riesgo de sangrado u otros factores, revise los riesgos de eventos tromboembólicos y sangrado en cada visita de control, y asegúrese que todas las evaluaciones y decisiones estén
documentadas.
BPC 6:
Para los pacientes que toman anticoagulación, revise al menos anualmente la necesidad y la calidad de anticoagulación, o más frecuentemente si ocurren eventos clínicamente relevantes que afecten la anticoagulación o el riesgo de sangrado.
3. Índice de riesgo de sangrado
Descargar PDF con el desarrollo de la pregunta.
Recomendación 1:
En pacientes con FA que están empezando o han empezado anticoagulación, sugerimos utilizar el índice HAS-BLED para evaluar el riesgo de sangrado.
(Recomendación fuerte a favor, Certeza de la evidencia: Muy baja)
BPC 1:
En pacientes con alto riesgo de sangrado, brinde anticoagulación y controle los factores que aumenten dicho riesgo.
BPC 2:
Al discutir los beneficios y riesgos de la anticoagulación, explique al paciente que:
a. Para la mayoría de los pacientes, el beneficio de la anticoagulación sobrepasa al riesgo de sangrado.
b. Para los pacientes con riesgo de sangrado incrementado, el beneficio de la anticoagulación puede no siempre sobrepasar al riesgo de sangrado, por lo que es importante un monitoreo cuidadoso del riesgo de sangrado.
BPC 3:
No evite la anticoagulación solo por el riesgo de caídas.
4. Oclusión de orejuela de aurícula izquierda
Descargar PDF con el desarrollo de la pregunta.
Recomendación 1:
Si existe alguna contraindicación absoluta para el uso de anticoagulantes a largo plazo, sugerimos considerar la OOAI.
(Recomendación condicional a favor, Certeza de la evidencia: Muy baja)
BPC 1:
Cuando se considere realizar OOAI, discutir sus beneficios y riesgos con el paciente.
5. Estrategias de control de ritmo vs estrategias de control de frecuencia
Descargar PDF con el desarrollo de la pregunta.
Recomendación 1:
Para la terapia de mantenimiento en pacientes con FA, sugerimos brindar control de la frecuencia como la estrategia de primera línea.
(Recomendación condicional a favor, Certeza de la evidencia: Muy baja)
BPC 1:
En pacientes con FA, considere brindar estrategias de control de ritmo cuando cumpla con alguna de las siguientes características:
a. FA de reciente inicio (menor de 48 horas).
b. Presencia de síntomas (escala EHRA) a pesar de que la frecuencia cardiaca está controlada o en quienes la estrategia de control de frecuencia no ha sido exitosa.
c. Presenta insuficiencia cardíaca que se sospecha es causada principalmente por la FA.
d. Es decidido en función del juicio clínico, considerando: tiempo de aparición menor de un año, crecimiento auricular no severo y ausencia de fragilidad.
6. Estrategias de control de frecuencia
Descargar PDF con el desarrollo de la pregunta.
Recomendación 1:
En pacientes con FA en quienes se elija una estrategia de control de frecuencia a largo plazo, sugerimos utilizar betabloqueadores o bloqueadores de canales de calcio no dihidropiridínicos.
(Recomendación fuerte a favor, Certeza de la evidencia: Muy baja)
BPC 1:
En pacientes con FA en quienes se elija una estrategia de control de frecuencia, ofrezca un betabloqueador estándar como monoterapia inicial.
BPC 2:
En pacientes con FA en quienes se elija una estrategia de control de frecuencia y tengan FEVI < 40%, no utilizar bloqueadores de los canales de calcio no dihidropiridínico (Verapamilo o Diltiazem).
BPC 3:
En pacientes sedentarios con FA no paroxística en quienes se elija una estrategia de control de frecuencia, puede considerar la
monoterapia con digoxina.
BPC 4:
En pacientes con FA, si la monoterapia no cesa los síntomas debido al control deficiente de la frecuencia ventricular, considere brindar terapia combinada con cualquiera de los siguientes:
a. Betabloqueador
b. Bloqueador de canales de calcio no dihidropiridínicos (Verapamilo o Diltiazem)
c. Digoxina
BPC 5:
En pacientes con FA, no ofrezca amiodarona para control de frecuencia a largo plazo.
BPC 6:
En pacientes con FA, elija el tratamiento farmacológico considerando los síntomas, la frecuencia cardíaca, las comorbilidades y las preferencias del paciente.
BPC 7:
En pacientes con FA permanente muy sintomáticos, en quienes el tratamiento farmacológico ha fracasado o no es tolerado, o presentan síntomas de disfunción ventricular izquierda que se sospecha son originados por la respuesta ventricular alta, considerar la ablación del nodo auriculoventricular y estimulación cardiaca (marcapasos o resincronizador).
7. Estrategias de control de ritmo
Descargar PDF con el desarrollo de la pregunta.
Recomendación 1:
En pacientes con FA en quienes se elija una estrategia de control de ritmo a largo plazo, sugerimos brindar un betabloqueador (excepto sotalol) como tratamiento inicial.
(Recomendación condicional a favor, Certeza de la evidencia: Muy baja)
BPC 1:
Si los betabloqueadores están contraindicados o no tienen éxito, evalúe la idoneidad de los fármacos alternativos disponibles para el control del ritmo (antiarritmicos clase Ic o III), teniendo en cuenta las comorbilidades.
Recomendación 2:
En pacientes con FA en quienes se elija una estrategia de control de ritmo a largo plazo, sugerimos no brindar fármacos antiarrítmicos de clase Ic como propafenona a personas con cardiopatía conocida.
BPC 1:
Considere la amiodarona en personas con disfunción ventricular izquierda o insuficiencia cardíaca.
BPC 2:
Para personas con FA de más de 48 horas de duración en quienes está indicada la cardioversión electiva, la cardioversión guiada por ecocardiografía transesofágica (ETE) o la cardioversión convencional (sin ETE y rangos óptimos de anticoagulación oral) deben considerarse igualmente seguras.
8. Ablación mediante cateterismo
Descargar PDF con el desarrollo de la pregunta.
Recomendación 1:
En pacientes con FA paroxística sintomática, luego de valorar la factibilidad y aceptabilidad de esta intervención, sugerimos realizar ablación mediante cateterismo en alguno de los siguientes escenarios:
• Refractarios o intolerantes a al menos un fármaco
antiarrítmico clase 1 o 3
• Refractarios o intolerantes a betabloqueadores.
(Recomendación condicional a favor**,Certeza de la evidencia: Muy baja)
Recomendación 2:
En pacientes con FA persistente sintomática, luego de valorar la factibilidad y aceptabilidad de esta intervención, sugerimos realizar ablación mediante cateterismo en alguno de los siguientes escenarios:
• Refractarios o intolerantes a al menos un fármaco
antiarrítmico clase 1 o 3
• Refractarios o intolerantes a betabloqueadores.
(Recomendación condicional a favor**, Certeza de la evidencia: Muy baja)
Recomendación 3:
En pacientes con FA sintomática asociada a insuficiencia cardiaca crónica con FE reducida < 35% en terapia médica óptima, luego una valoración individualizada de los beneficios y daños, sugerimos realizar ablación mediante cateterismo.
(Recomendación condicional a favor**, Certeza de la evidencia: Muy baja)
BPC 1:
En pacientes con FA y disfunción sistólica del VI, con alta sospecha de taquicardiomiopatía asociada a FA, considerar realizar ablación mediante cateterismo.
BPC 2:
En pacientes con síndrome taquicardia bradicardia documentada como FA seguida de una pausa significativa, considerar realizar ablación mediante cateterismo para reducir los síntomas de la FA y disminuir la necesidad de implantación de marcapasos.
BPC 3:
En pacientes con FA sintomática (paroxística o persistente), considere la ablación quirúrgica concomitante a otra cirugía cardiotorácica.
9. Cardioversión eléctrica de emergencia
Descargar PDF con el desarrollo de la pregunta.
Recomendación 1:
En pacientes con FA con respuesta ventricular rápida en quienes se detecte inestabilidad hemodinámica, recomendamos usar cardioversión eléctrica de emergencia.
(Recomendación fuerte a favor, Certeza de la evidencia: Muy baja)
BPC 1:
En pacientes con FA de más de 48 horas de duración que se encuentren hemodinámicamente estables, en quienes se considere estrategia de control de ritmo, considerar la realización de cardioversión guiada por ecocardiografía
transesofágica (ETE).
10. Cardioversión farmacológica autoadministrada (pill in the pocket)
Descargar PDF con el desarrollo de la pregunta.
BPC 1:
En pacientes con FA de presentación aguda, considerar administrar la estrategia “Pill in the pocket” con propafenona en dosis de carga (450-600 mg) para aquellos pacientes con FA paroxística que cumplen con todos los siguientes criterios:
a. Cuando esta terapia ha sido efectiva al ser administrada bajo monitoreo médico.
b. No tienen antecedentes de disfunción ventricular izquierda, o enfermedad cardíaca valvular significativa o isquémica.
c. Tener un historial de episodios sintomáticos infrecuentes (no más de 6 episodios por año) de FA paroxística.
d. Tener una presión arterial sistólica mayor a 100 mmHg y una frecuencia cardíaca en reposo por encima de 70 lpm.
e. Ser capaces de entender cómo y cuándo tomar el medicamento.
Referencias bibliográficas
1. Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, et al. Prostate cancer. Nature reviews Disease primers. 2021;7(1):9.
2. Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG. Prostate cancer. Lancet (London, England). 2021;398(10305):1075-90.
3. Sayegh N, Swami U, Agarwal N. Recent Advances in the Management of Metastatic Prostate Cancer. JCO oncology practice. 2022;18(1):45-55.
4. Ng K, Smith S, Shamash J. Metastatic Hormone-Sensitive Prostate Cancer (mHSPC): Advances and Treatment Strategies in the First-Line Setting. Oncology and therapy. 2020;8(2):209-30.
5. Shiota M, Terada N, Saito T, Yokomizo A, Kohei N, Goto T, et al. Differential prognostic factors in low- and high-burden de novo metastatic hormone-sensitive prostate cancer patients. Cancer science. 2021;112(4):1524-33.
6. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2010;182(18):E839-42.
7. Ministerio de Salud. Documento técnico: Metodología para la de documento técnico elaboración guías de practica clínica. Lima PM.
8. Virgo KS, Rumble RB, de Wit R, Mendelson DS, Smith TJ, Taplin ME, et al. Initial Management of Noncastrate Advanced, Recurrent, or Metastatic Prostate Cancer: ASCO Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2021;39(11):1274-305.
9. EAU Guidelines Office. Prostate Cancer. Arnhem (The Netherlands): EAU Guidelines Office; 2021. [citado el 20 de mayo de 2022]. Edición 2021. Disponible en: https://uroweb.org/guidelines/prostate-cancer.
10. National Institute for Health and Care Excellence (NICE). Prostate cancer: diagnosis and management. London: NICE; 2019 [citado el 20 de mayo de 2022]. Disponible en: https://www.nice.org.uk/guidance/ng131.
11. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed). 2017;358:j4008.
12. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2011;343:d5928.
13. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle- Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta- analyses. Oxford; 2000.
14. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS- 2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155(8):529-36.
15. Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. Journal of clinical epidemiology. 2013;66(7):726-35.
16. Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ (Clinical research ed). 2016;353:i2016.
17. Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ (Clinical research ed). 2016;353:i2089.
18. Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of clinical epidemiology. 2013;66(7):719-25. 19.Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long- Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology.
2018;36(11):1080-7.
20. Liu M, Qu S, Liu Y, Yao X, Jiang W. Comparative clinical effects and cost-effectiveness of maximum androgen blockade, docetaxel with androgen deprivation therapy and ADT alone for the treatment of mHSPC in China. Journal of comparative effectiveness research. 2019;8(11):865-77.
21. Yang Y, Chen R, Sun T, Zhao L, Liu F, Ren S, et al. Efficacy and safety of combined androgen blockade with antiandrogen for advanced prostate cancer. Current oncology (Toronto, Ont). 2019;26(1):e39-e47.
22. Wu J, Chen WK, Zhang W, Zhang JS, Liu JH, Jiang YM, et al. Network meta-analysis of the efficacy and adverse effects of several treatments for advanced/metastatic prostate cancer. Oncotarget. 2017;8(35):59709-19.
23. Samson DJ, Seidenfeld J, Schmitt B, Hasselblad V, Albertsen PC, Bennett CL, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer. 2002;95(2):361-76.
24. Schmitt B, Wilt TJ, Schellhammer PF, DeMasi V, Sartor O, Crawford ED, et al. Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: a systematic review. Urology. 2001;57(4):727-32.
25. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists’ Collaborative Group. Lancet (London, England). 2000;355(9214):1491-8.
26. Bennett CL, Tosteson TD, Schmitt B, Weinberg PD, Ernstoff MS, Ross SD. Maximum androgen-blockade with medical or surgical castration in advanced prostate cancer: A meta-analysis of nine published randomized controlled trials and 4128 patients using flutamide. Prostate cancer and prostatic diseases. 1999;2(1):4-8.
27. Seidenfeld J, Samson DJ, Aronson N, Albertson PC, Bayoumi AM, Bennett C, et al. Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evidence report/technology assessment (Summary). 1999(4):i-x, 1-246, I1-36, passim.
28. Caubet JF, Tosteson TD, Dong EW, Naylon EM, Whiting GW, Ernstoff MS, et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology. 1997;49(1):71-8.
29. Bubley GJ. Is the flare phenomenon clinically significant? Urology. 2001;58(2 Suppl 1):5-9.
30. Kunath F, Jensen K, Pinart M, Kahlmeyer A, Schmidt S, Price CL, et al. Early versus deferred standard androgen suppression therapy for advanced hormone-sensitive prostate cancer. The Cochrane database of systematic reviews. 2019;6(6):Cd003506.
31. Seidenfeld J, Samson DJ, Hasselblad V, Aronson N, Albertsen PC, Bennett CL, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Annals of internal medicine. 2000;132(7):566-77. 32.Sun M, Choueiri TK, Hamnvik OP, Preston MA, De Velasco G, Jiang W, et al. Comparison of Gonadotropin-Releasing Hormone Agonists and Orchiectomy: Effects of
Androgen-Deprivation Therapy. JAMA oncology. 2016;2(4):500-7.
33. Magnan S, Zarychanski R, Pilote L, Bernier L, Shemilt M, Vigneault E, et al. Intermittent vs Continuous Androgen Deprivation Therapy for Prostate Cancer: A Systematic Review and Meta-analysis. JAMA oncology. 2015;1(9):1261-9.
34. Brungs D, Chen J, Masson P, Epstein RJ. Intermittent androgen deprivation is a rational standard-of-care treatment for all stages of progressive prostate cancer: results from a systematic review and meta-analysis. Prostate cancer and prostatic diseases. 2014;17(2):105-11.
35. Botrel TE, Clark O, dos Reis RB, Pompeo AC, Ferreira U, Sadi MV, et al. Intermittent versus continuous androgen deprivation for locally advanced, recurrent or metastatic prostate cancer: a systematic review and meta-analysis. BMC urology. 2014;14:9.
36. Tsai HT, Penson DF, Makambi KH, Lynch JH, Van Den Eeden SK, Potosky AL. Efficacy of intermittent androgen deprivation therapy vs conventional continuous androgen deprivation therapy for advanced prostate cancer: a meta-analysis. Urology. 2013;82(2):327-33.
37. Weiner AB, Siebert AL, Fenton SE, Abida W, Agarwal N, Davis ID, et al. First-line Systemic Treatment of Recurrent Prostate Cancer After Primary or Salvage Local Therapy: A Systematic Review of the Literature. European urology oncology. 2022;5(4):377-87.
38. Menges D, Yebyo HG, Sivec-Muniz S, Haile SR, Barbier MC, Tomonaga Y, et al. Treatments for Metastatic Hormone-sensitive Prostate Cancer: Systematic Review, Network Meta-analysis, and Benefit-harm assessment. European urology oncology. 2022;5(6):605-16.
39. Mori K, Mostafaei H, Sari Motlagh R, Pradere B, Quhal F, Laukhtina E, et al. Systemic therapies for metastatic hormone-sensitive prostate cancer: network meta-analysis. BJU international. 2022;129(4):423-33.
40. Wenzel M, Würnschimmel C, Nocera L, Collà Ruvolo C, Tian Z, Shariat SF, et al. Overall Survival After Systemic Treatment in High-volume Versus Low-volume Metastatic Hormone-sensitive Prostate Cancer: Systematic Review and Network Meta-analysis. European urology focus. 2022;8(2):399-408.
41. Wang L, Paller CJ, Hong H, De Felice A, Alexander GC, Brawley O. Comparison of Systemic Treatments for Metastatic Castration-Sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. JAMA oncology. 2021;7(3):412-20.
42. Ferro M, Lucarelli G, Crocetto F, Dolce P, Verde A, La Civita E, et al. First-line systemic therapy for metastatic castration-sensitive prostate cancer: An updated systematic review with novel findings. Critical reviews in oncology/hematology. 2021;157:103198.43. Wang Y, Gui H, Wang J, Tian J, Wang H, Liang C, et al. Comparative Efficacy of Combined Radiotherapy, Systemic Therapy, and Androgen Deprivation Therapy for Metastatic Hormone-Sensitive Prostate Cancer: A Network Meta-Analysis and Systematic Review. Frontiers in oncology. 2020;10:567616.
44. Chen J, Ni Y, Sun G, Liao B, Zhang X, Zhao J, et al. Comparison of Current Systemic Combination Therapies for Metastatic Hormone-Sensitive Prostate Cancer and Selection of Candidates for Optimal Treatment: A Systematic Review and Bayesian Network Meta-Analysis. Frontiers in oncology. 2020;10:519388.
45. Sathianathen NJ, Koschel S, Thangasamy IA, Teh J, Alghazo O, Butcher G, et al. Indirect Comparisons of Efficacy between Combination Approaches in Metastatic Hormone- sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. European urology. 2020;77(3):365-72.
46. Sathianathen NJ, Philippou YA, Kuntz GM, Konety BR, Gupta S, Lamb AD, et al. Taxane- based chemohormonal therapy for metastatic hormone-sensitive prostate cancer: a Cochrane Review. BJU international. 2019;124(3):370-2.
47. Sun G, Zhang X, Chen J, Liao B, Liu Z, Zhao J, et al. What kind of patients with castration-naïve prostate cancer can benefit from upfront docetaxel and abiraterone: A systematic review and a network meta-analysis. Urologic oncology. 2018;36(12):505- 17.
48. Tan PS, Aguiar P, Jr., Haaland B, Lopes G. Addition of abiraterone, docetaxel, bisphosphonate, celecoxib or combinations to androgen-deprivation therapy (ADT) for metastatic hormone-sensitive prostate cancer (mHSPC): a network meta-analysis. Prostate cancer and prostatic diseases. 2018;21(4):516-23.
49. Vale CL, Fisher DJ, White IR, Carpenter JR, Burdett S, Clarke NW, et al. What is the optimal systemic treatment of men with metastatic, hormone-naive prostate cancer? A STOPCAP systematic review and network meta-analysis. Annals of oncology : official journal of the European Society for Medical Oncology. 2018;29(5):1249-57.
50. Gravis G, Boher JM, Chen YH, Liu G, Fizazi K, Carducci MA, et al. Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. European urology. 2018;73(6):847-55.
51. Clarke NW, Ali A, Ingleby FC, Hoyle A, Amos CL, Attard G, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Annals of oncology : official journal of the European Society for Medical Oncology. 2019;30(12):1992-2003.
52. Gravis G, Boher JM, Joly F, Soulié M, Albiges L, Priou F, et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel Versus ADT Alone in Metastatic Non castrate Prostate Cancer: Impact of Metastatic Burden and Long-term Survival Analysis of the Randomized Phase 3 GETUG-AFU15 Trial. European urology. 2016;70(2):256-62.
53. Burdett S, Boevé LM, Ingleby FC, Fisher DJ, Rydzewska LH, Vale CL, et al. Prostate Radiotherapy for Metastatic Hormone-sensitive Prostate Cancer: A STOPCAP Systematic Review and Meta-analysis. European urology. 2019;76(1):115-24.
54. Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet (London, England). 2018;392(10162):2353- 66.
55. Boevé LMS, Hulshof M, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. European urology. 2019;75(3):410-8.
56. Instituto de Evaluación de Tecnologías en Salud e Investigación (IETSI). Resolución N°12-IETSI-2023. Lima: EsSalud.; 2023 [citado el 21 de mayo de 2023]. Disponible en: https://ietsi.essalud.gob.pe/wp-content/uploads/2023/02/RESOLUCION-N%C2%B0-
12-IETSI-2023-APREPITANT-125MG-Y-ACETATO-DE-ABIRATERONA-250MG.pdf.
Si tienes comentarios sobre el contenido de las guías de práctica clínica, puedes comunicarte con IETSI-EsSalud enviando un correo: gpcdireccion.ietsi@essalud.gob.pe
SUGERENCIAS
Si has encontrado un error en esta página web o tienes alguna sugerencia para su mejora, puedes comunicarte con EviSalud enviando un correo a evisalud@gmail.com


